Abstract
INTRODUCTİON Glofitamab is a T-cell-engaging bispecific antibody connecting CD20 on B cells and CD3 on T cells. Although, most of the patients with B-cell non-Hodgkin lymphoma (BNHL) achieve complete response (CR) following firstline treatment with rituximab and chemotherapy, about 40% of patients with diffuse large B-cell lymphoma (DLBCL) is refractory or relapse (R/R). Autologous stem-cell transplantation (ASCT) can cure some of these patients but many patients cannot undergo this procedure. CAR-T therapies are a significant advance but not available in many countries like Turkey. In Phase II expansion study, the overall response rate (ORR) was 51.6% and complete remission (CR) rate was 39.4% in R/R DLBCL patients (Dickinson er al. JCO 2022). In this retrospective study, we aimed to report the outcomes of patients who used glofitamab via compessionate use in Turkey.
METHOD Glofitamab is available via compassionate use in Turkey for patients >18 years and with relapsed/refractory Diffuse Large B Cell Lymphoma (DLBCL), transformed Follicular Lymphoma (tFL) and Primary Mediastinal B Cell Lymphoma(PMBCL) who had received 3 lines of treatment previously. Patients received 1000mg obinutuzumab 7 days prior to first dose of glofitamab. Glofitamab was given intravenously at a fixed dose
2.5/10/30mg on Cycle (C) 1 Day (D) 1and 8, and then at the target dose from C2D1 q3w, for up to 12 cycles. Response rates are based on Lugano criteria (Cheson et al. JCO 2014). Response evaluations were done by fluorodeoxyglucose positron emission tomography and computed tomography (PET/CT) after the 2nd-4th cycles at the discretion of the treating physician.
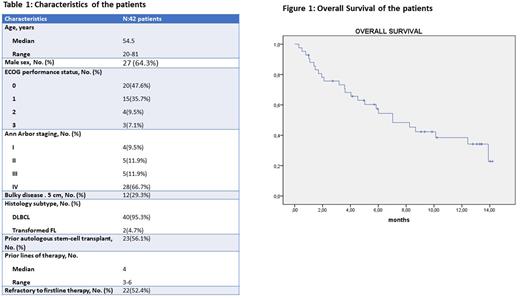
RESULTS As of July 1st, 2022,46 patinets used Glofitamab on compessionate use. The results of 42 pts who at least used glofitamab once are represented here. Median age was 54.5 (range, 20‒81) years, 64.3% were male, and the median lines of prior therapies was 4 (range, 3‒6). Except 2 patients who had transformed Follicular lymphoma, the rest 40 patients had Diffuse Large B cell Lymphoma. The characteristics of the patients are shown in Table 1. The patients received median 4 cycles (1-12) of Glofitamab. Seven patients (16,7%) died before response assessment. In efficacy-evaluable patients, the ORR rate was 28.5 % (12 patients) and the CR rate was 19 %(8 pts). While, 3 patients (7.1 %) had stable disease (SD), 47.6 % of patients (20 patients) had progressive disease after Glofitamab treatment. A total of 5 patients proceeded to stem cell transplantation after Glofitamab treatment (3 allogeneic stem cell transplantation (AlloSCT), 2 ASCT). The most commonly encountered toxicities were hematological; neutropenia was observed in 41.5% of patients and in 23% it was ≥grade 3, anemia was observed in 38.1% of patients and in 19% it was ≥grade3, thrombocytopenia was observed in 28.6% of patients and in 19% it was ≥19%. Cytokine Release Syndrome(CRS) was encountered in 12 patients (28.6%), in 4 patients it was ≤grade2 but in the rest 4 patients it was ≥grade3. Neurological toxicity was observed in only 3 patients and in all of them it was ≤grade2. This compessionate use program was conducted in the COVID19 pandemic era and 9 patients (21.4%) had COVID19 infection during treatment. The median follow-up was 5,78 months (range: 0,30-14,19 ). The median overall survival was 7 months (95% CI: 4.02‒10.03) (Figure 1). Seventeen patients were alive and 25 patients had died at the time of analysis. Five patients died due to COVID19 infection, 1 patient died early in the 1st cycle due to unknown causes, 2 patients who proceeded to AlloSCT died due to transplantation related complications, 3 patients died due to sepsis and the rest of the patients died due to disease progression.
CONCLUSION To our knowledge, this is the largest real world data on the effectiveness and toxicity of Glofitamab treatment in R/R DLBCL patients. The response rates are lower when compared to Phase I study, but in this study the patients were more heavily pretreated and more than half of the patients had received ASCT previously and were refractory to firstline therapy. Seven months median OS seems to be promising in this heavily pretreated group and moreover 5 patients (12%) who died due to COVID19 infection and 2 patients who died due to transplantation related complications after AlloSCT should also be considered.
Disclosures
Özcan:Janssen: Research Funding; Reddy's: Research Funding; Acerta: Research Funding; Bayer: Research Funding; MSD: Research Funding; Roche: Other: Travel expenses/accommodations, Research Funding; Takeda: Research Funding; Abbvie: Other: Travel expenses/accommodations, Research Funding; Pfizer: Research Funding; Jazz: Other: Travel expenses/accommodations.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal